
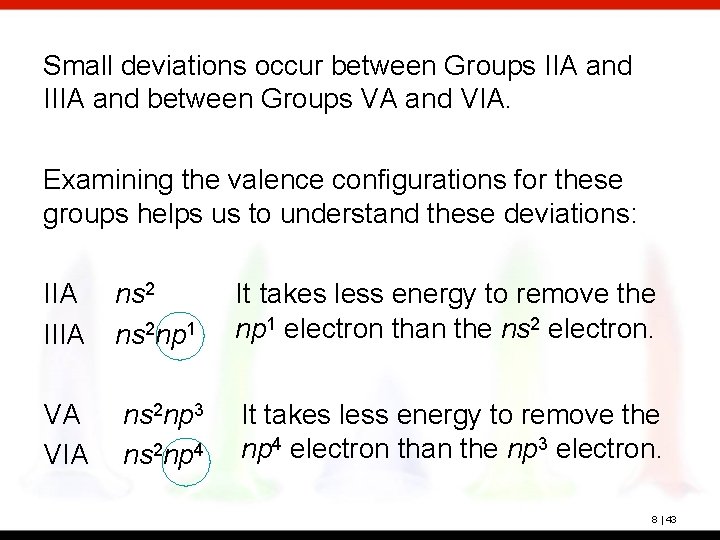
The elements with the lowest atomic number that has a ground state electronic configuration of (n 1) d6ns2 is located in the:a)Fifth periodb)Sixth periodc)Fourth periodd)Third periodCorrect answer is option 'C'. You can study other questions, MCQs, videos and tests for Chemistry on EduRev and even discuss your questions like
If the answer is not available please wait for a while and a community member will probably answer this Explore molecule shapes by building molecules in 3D How does molecule shape change with different numbers of bonds and electron pairs Find out by adding single, double or triple bonds and lone pairs to the central atom. (iii) Elements with this electron configuration are nonmetals. The general electronic outermost configuration of noble gases (except for He) is ns2 np6. Consider the general valence electron configuration of ns2np5 and the following statements: (i) Elements with this electron configuration are expected to form -1 anions. ns2 np6 The outermost electronic configuration of the most electronegative element is a. Noble Gases: Group 18 gaseous elements are called noble gasses. The outermost electronic configuration of the most electronegative element is a. Valence electrons are those that are in the highest principal energy level. How Many Valence Electrons Does An Oxygen Atom Have Chemistry.

The Electron Configuration Of An Oxygen Atom Is 1s22s22p4.
#ATOM WITH NS2 ELECTRON CONFIGURATION FULL#
The outer electronic configuration is characteristic of (n-2) f1-14 (n-1) d0-1 ns2. A full octet of electrons, this means that the highest energy level has 8 electrons.

Can you explain this answer? are solved by group of students and teacher of Chemistry, which is also the largest studentĬommunity of Chemistry. Actinoids distinguished by 5f-orbitals filling are the elements from Th 103Lr following actinium. The Questions andĪnswers of The elements with the lowest atomic number that has a ground state electronic configuration of (n 1) d6ns2 is located in the:a)Fifth periodb)Sixth periodc)Fourth periodd)Third periodCorrect answer is option 'C'. Can you explain this answer? is done on EduRev Study Group by Chemistry Students. This discussion on The elements with the lowest atomic number that has a ground state electronic configuration of (n 1) d6ns2 is located in the:a)Fifth periodb)Sixth periodc)Fourth periodd)Third periodCorrect answer is option 'C'.


 0 kommentar(er)
0 kommentar(er)
